

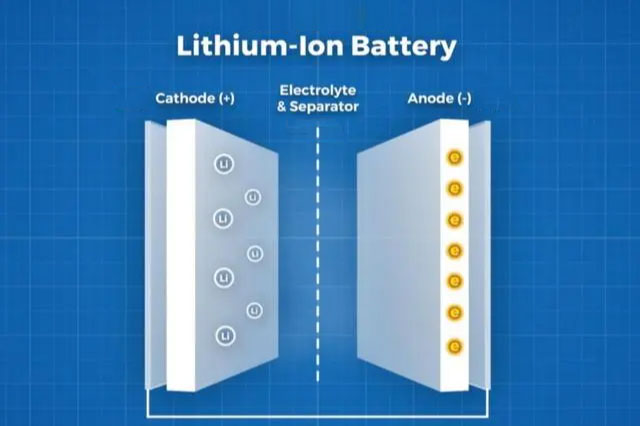
The working principle of lithium batteries is developed by Utilizing the characteristics of "lithium ions can carry electrons". Lithium batteries are generally made of "lithium compounds" and "carbon materials", and lithium ions can be intercalated or deintercalated in these two materials. During the process of this phenomenon, electrons will also migrate with lithium ions. This process can be understood as the process of battery charging and discharging. The internal structure, material, and applied technology are different, and this will determine the overall performance of the battery, especially the lithium battery.

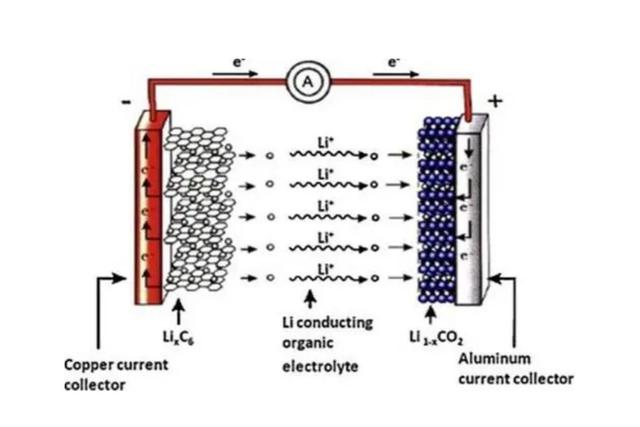
The battery bulging phenomenon that occurs in daily life is also related to the shuttle of lithium ions. If lithium ions carry a large number of electrons to the negative electrode area, if these charged lithium ions cannot be stored, overcharging and bulging will occur. Otherwise, it will be overdischarging. Although the direction is positive and negative, the principle is the same.
